Executive Summary
Filtration is the primary defense in laboratory ventilation systems, particularly in biosafety level 2 (BSL-2) and biosafety level 3 (BSL-3) environments where airborne biological hazards may be present. Prefilters and secondary filters are essential for capturing particulate matter, preventing environmental contamination, and preserving the efficiency and integrity of high-efficiency particulate air (HEPA) filters. However, their performance depends on consistent and rigorous maintenance.
This white paper outlines the role of filters within laboratory biosafety systems, examines the risks associated with neglected maintenance, and presents a framework for inspection, replacement, and documentation practices aligned with biosafety standards.
1. Introduction
Laboratory ventilation systems are designed to control airborne contaminants, maintain directional airflow, and ensure biosafety containment. These systems rely on multi-stage filtration to remove particulate matter and protect both personnel and the surrounding environment.
High-efficiency particulate air (HEPA) filters, which remove 99.97% of particles ≥0.3 microns, are essential components in BSL-2 and BSL-3 laboratories. In biological safety cabinets (BSCs), HEPA filters are standard, although some Class II and Class III BSCs incorporate ultra-low penetration air (ULPA) filters, which provide even finer filtration down to 0.1 microns at 99.999% efficiency. These filters ensure that work zones and exhaust air are free of viable biological agents.
The performance of HEPA and ULPA filters is supported by prefilters and secondary filters that protect the system and extend operational lifespan. Effective maintenance of all filtration stages is critical to sustaining compliance with international biosafety regulations and to preventing performance degradation that could compromise safety or disrupt operations.
2. Filter Types and Functions in Laboratory Ventilation
2.1 Prefilters
Prefilters remove larger particulates such as dust and debris before air reaches downstream filters. They reduce system load and help prevent premature fouling of secondary and HEPA filters. Typically located at air intake points or upstream in supply ducts, prefilters are the first line of defense in multi-stage systems.
2.2 Secondary Filters
Secondary filters are placed between prefilters and the terminal HEPA stage. They capture finer particulates and provide redundancy in environments with elevated dust loads or specific air quality challenges. Their use increases the efficiency and extends the life of HEPA filters, especially in facilities that operate in harsh or remote environments.
2.3 HEPA Filters
HEPA filters are installed in biosafety cabinets and, in BSL-3 laboratories, are also found in supply and exhaust ducts as determined by the facility’s risk assessment. They are the final filtration barrier, preventing the escape of viable biological agents into the environment and ensuring that recirculated or discharged air meets biosafety requirements.
Smoke test caried out during validation
3. Consequences of Poor Filter Maintenance
3.1 Loss of Containment
Neglected or compromised filters can lead to airflow disruption and loss of directional pressure gradients. In BSL-3 environments, this undermines containment integrity and may allow particulates or pathogens to bypass the filtration system, especially in biological safety cabinets.
3.2 Equipment Strain and System Failure
Clogged filters increase resistance within the HVAC system. This leads to excessive fan loading, higher energy consumption, and potential system failure. Over time, this results in costly repairs and unscheduled downtime.
3.3 Non-Compliance with Biosafety Standards
International biosafety frameworks—including the CDC/NIH Biosafety in Microbiological and Biomedical Laboratories (BMBL), WHO Laboratory Biosafety Manual (LBM), and EN 12469—require scheduled testing, maintenance, and documentation of filter integrity. Failure to comply with these standards can result in certification lapses or regulatory penalties.
3.4 Operational Interruptions
Filter failures, especially when detected during audits or operational incidents, often require immediate shutdown of laboratory activities. This can delay critical diagnostics, research, or response efforts and cause significant reputational and financial damage.
4. Maintenance Best Practices
4.1 Inspection Frequency
Visual Inspection: Prefilters and secondary filters should be visually inspected monthly or as specified by the manufacturer.
HEPA Integrity Testing: Conduct annually or following any event that may compromise filter integrity. Use aerosol challenge methods such as DOP or PAO testing.
Pressure Drop Monitoring: Use continuous or periodic monitoring across each filtration stage to detect clogging or airflow restriction.
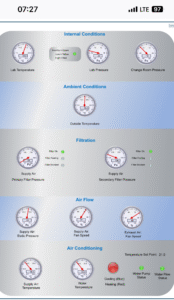
Remote monitoring system display indicating system health in a BSL-3 laboratory.
4.2 Replacement Protocols
Prefilters and Secondary Filters: Replace when visibly soiled or when pressure drop exceeds predefined thresholds (t

Prefilter replaced with new filter.
ypically every 3 to 6 months).

Fouled prefilter removed for replacement.
HEPA Filters: Replace every 3 to 5 years, or sooner if integrity is compromised or pressure resistance is excessive.
HEPA Change Procedures: Must follow approved biosafety protocols including use of PPE, decontamination procedures, and secure waste handling.
4.3 Documentation and Traceability
Maintain a complete record of all inspections, test results, filter replacements, and service activities. These logs are essential for internal quality assurance and must be available for review during regulatory audits or certifications.
5. Conclusion
Filter maintenance is a critical component of laboratory biosafety infrastructure. Although often treated as a routine task, its role in protecting personnel, preserving facility integrity, and ensuring compliance is fundamental. Institutions operating BSL-2 and BSL-3 laboratories must integrate rigorous filter maintenance protocols into their operational procedures. These practices support not only the reliability and longevity of laboratory systems but also their readiness to respond to public health and research demands without interruption.
6. About LIS-Labs
LIS-Labs designs and deploys mobile and modular BSL-2 and BSL-3 laboratories engineered for reliability, resilience, and regulatory compliance. Our laboratory systems feature multi-stage ventilation and filtration systems with integrated access for service and maintenance. Remote monitoring tools allow real-time assessment of filter loading, and our support services include inspection protocols, HEPA integrity testing, and maintenance documentation systems.
We help clients ensure peak laboratory performance from commissioning to field operation.
Contact: www.lis-labs.com/contact-us/
Website: https://www.lis-labs.com


