Executive Summary
Ventilation systems are the primary engineering control in high containment laboratories. They manage airborne hazards, prevent environmental contamination, and protect personnel. This white paper explains how ventilation supports design, and operation, with emphasis on airflow control, pressure cascading, filtration, and regulatory compliance.
1. Introduction
Ventilation is both a functional necessity and a statutory requirement. BSL 2 laboratories work with moderately hazardous agents, while BSL 3 laboratories handle airborne pathogens that can cause serious or fatal disease. Effective ventilation underpins containment, personnel safety, and operational reliability in both settings.
2. Core Ventilation Requirements by Biosafety Level
BSL 2 Laboratory Ventilation
Air changes: 6 – 12 air changes per hour (ACH) are recommended.
Containment: Class II biosafety cabinets are mandatory for aerosol generating work.
System approach: Room ventilation connects to the building HVAC but must be managed to avoid recirculating potentially contaminated air.
BSL 3 Laboratory Ventilation
Negative pressure: Air must always flow from clean to potentially contaminated areas.
Pressure zoning: A cascade of pressure differentials is maintained from support spaces into the containment core.
Dedicated ventilation: Supply and exhaust systems are isolated from other building areas and served by a dedicated HVAC system.
Air changes: A minimum of 12 ACH is required.
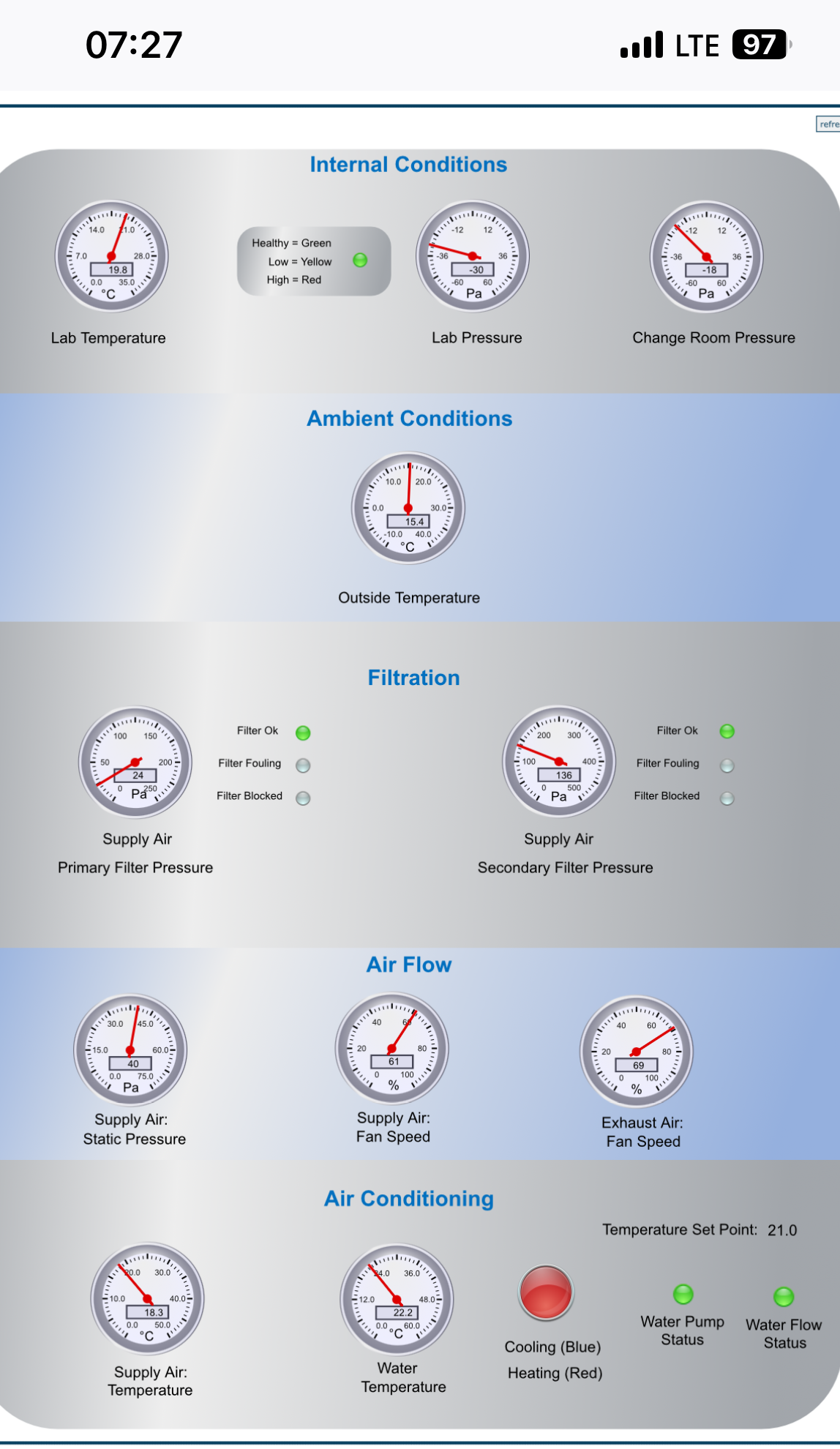
Monitoring and redundancy: Continuous pressure monitoring, alarms, backup and emergency power ensure uninterrupted operation.
3. Functional Objectives of Ventilation
Containment – Prevents pathogen escape during pressure or airflow fluctuations.
Personnel safety – Limits aerosol exposure during routine procedures.
Cross contamination control – Cascading of air in zones help safeguard clean areas and protects research integrity.
Environmental protection – HEPA filtered exhaust protects public health and the environment.
4. Design Considerations
System sizing and layout: Match airflow capacity to occupancy, heat loads, and room configuration; design duct routes and terminal locations to support proper flow.
System integration: Control ventilation with a building management system for real time alarms and diagnostics; maintain pressure cascade as filter loading occurs and ensure compatibility with biosafety cabinets and decontamination equipment.
Resilience: Allow for future upgrades, straightforward maintenance, and operation under design site environmental conditions.
5. Regulatory and Compliance Framework
Key references include:
WHO Laboratory Biosafety Manual (4th edition)
CDC/NIH Biosafety in Microbiological and Biomedical Laboratories (BMBL)
Early consideration of statutory and guideline requirements is essential during planning and design.
6. Commissioning and Validation
As part of factory acceptance testing or before occupancy:
Smoke visualisation of airflow patterns
Differential pressure verification
HEPA filter integrity testing (DOP/PAO)
Alarm functional checks
Air velocity measurements at critical points.Annual recertification is standard; high use facilities may require more frequent testing.

Smoke test to verify air flow patterns in a BSL-3 laboratory made by LIS.
7. Conclusion
Ventilation is a cornerstone of biosafety in BSL 2 and BSL 3 laboratories. Robust design, continuous monitoring, and proactive maintenance are indispensable for safe and reliable operation.
8. About LIS Labs
LIS Labs designs and delivers mobile and modular BSL 2 and BSL 3 laboratories that meet international standards. Each unit incorporates high performance ventilation and HEPA filtration, with options for solar or hybrid power to enable off grid deployment. Tailored maintenance agreements help minimise the risk of unforeseen failures.
Contact: https://www.lis-labs.com/contact-us/
Website: https://www.lis-labs.com